COVID-19 Vaccination Status
As outlined in the most recent release from the National Institutes of Health, a Phase 3 trial, designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 (COVID-19) in adults has begun. The vaccine, known as mRNA-1273, was co-developed by the Cambridge, Massachusetts-based biotechnology company Moderna, Inc., and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The trial, which will be conducted at U.S. clinical research sites, is expected to enroll approximately 30,000 adult volunteers who do not have COVID-19.
Phase 3 trials are:
- Conducted to confirm and expand on safety and effectiveness results from Phase I and Phase II trials
- Compare the drug to standard therapies for the disease or condition being studied
- Evaluate the overall risk and benefits of the drug
But while trials are starting each day and programs, such as Operation Warp Speed have goals of delivering 300 million doses of a safe, effective vaccine for COVID-19 by January 2021, public health experts warn of a long path ahead.
Vaccination Challenges
Aside from the vaccine manufacturing and distribution challenges, which will be expected, the American workforce may be hesitant to agree to vaccination.
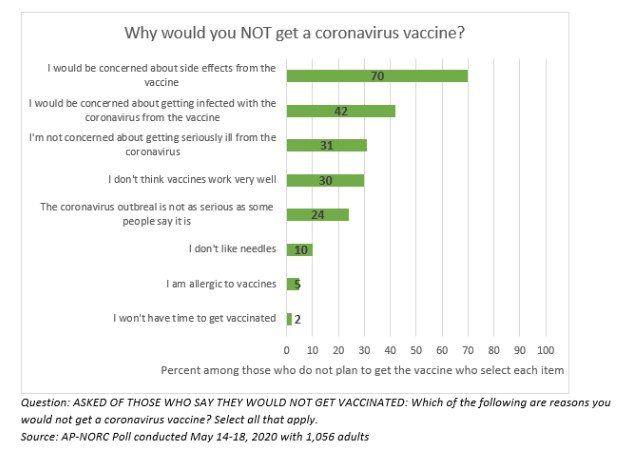
According to a study in May by the AP-NORC at the University of Chicago, if a vaccine against coronavirus becomes available to the public, 20% say they do not plan to get vaccinated with another 31% unsure.
Participants of the study were asked questions as to why they would not get the vaccine, to include:
- “I would be concerned about side effects from the vaccine”
- “I would be concerned about getting infected with the coronavirus from the vaccine”
- “I’m not concerned about getting seriously ill from the coronavirus”
- “I don’t think vaccines work very well”
- “The coronavirus outbreak is not as serious as some people say it is”
- “I don’t like needles”
- “I’m allergic to vaccines”
- “I won’t have to get vaccinated”
Among the 20% of Americans who say they will not get the vaccine, concern about side effects is overwhelmingly the top reason for avoiding the vaccine.
Best-Practice Focus
As advised by the World Health Organization this week, despite strong hopes for a vaccine, there might never be a ‘silver bullet’ for COVID-19. We have a long path ahead and our focus must remain on enforcement of health measures, such as:
- Mask-wearing
- Social distancing
- Hand-washing
- Testing
Though we all eagerly await an accessible COVID-19 vaccination, it, unfortunately, will not be the “quick fix” employers are hoping for, returning our workplaces to a sense of pre-pandemic normalcy. However, as the first to market with CheckIn2Work, our pre-screening app which flags potentially exposed and/or infected employees, followed by our case management service which closely monitors employees throughout their recovery journey and facilitates a successful reentry to the workplace, Axiom Medical is working closely with businesses across all industries to navigate this pandemic and beyond.

Holly is an ER nurse by trade, but loves content marketing. She was born outside the box and believes everything is better with “sprinkles and sparkles”. She is passionate about impacting lives and uses marketing as her platform for sharing practical solutions to address real life occupational health challenges.
Find out more about our Injury Case Management services or our Occupational Health Programs.